
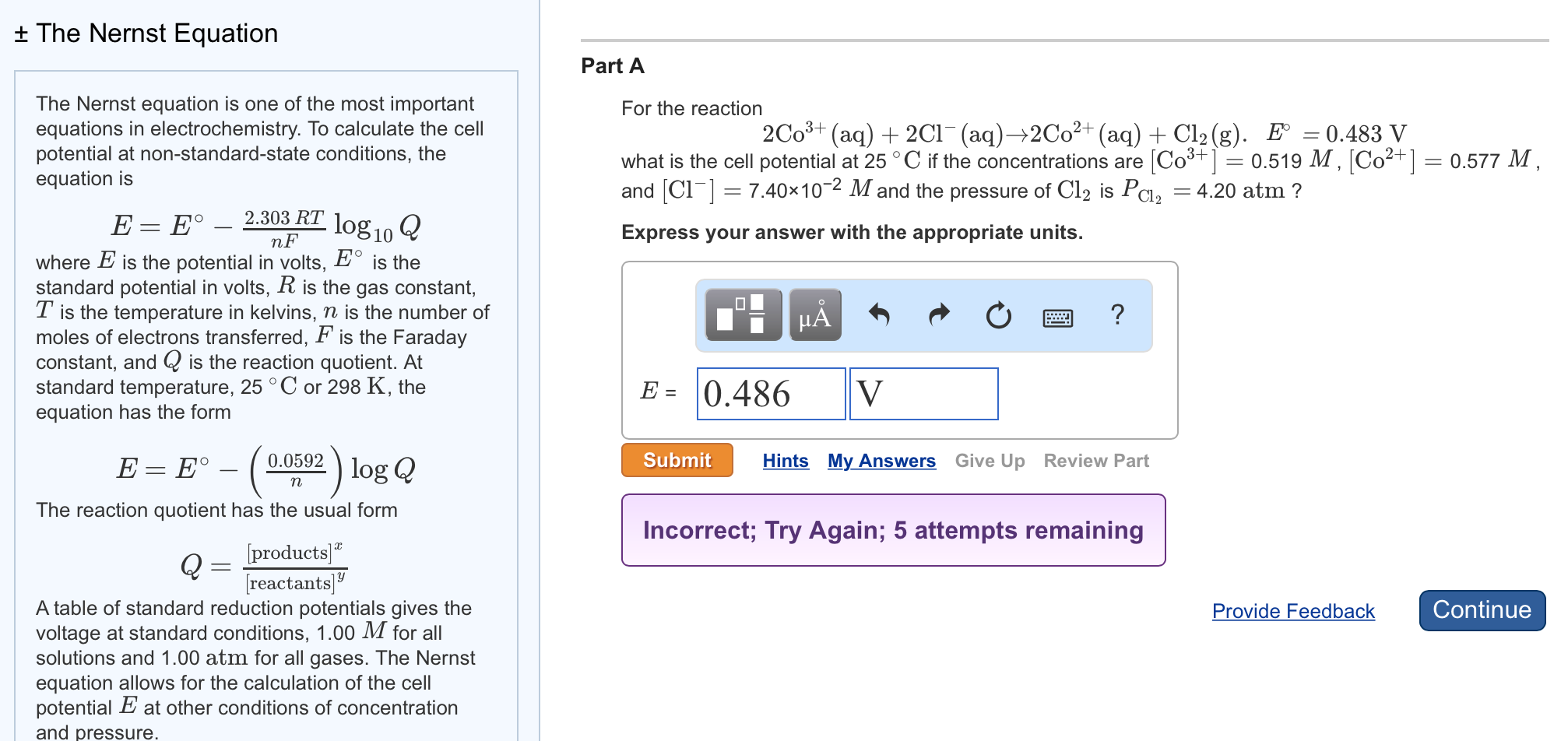
It relates the effective concentrations of the components of a cell reaction to the standard cell potential. It defines the relationship between cell potential to standard potential and to the activities of the electrically active species. The Nernst equation is an important relation which is used to determine reaction equilibrium constants and concentration potentials as well as to calculate the minimum energy required in electrodialysis. The Nernst equation is often used to calculate the cell potential of an electrochemical cell at any given temperature, pressure, and reactant concentration. And we essentially just change this from natural logarithm to base 10. So we can write the Nernst equation once again, alright, so E, or the cell potential, is equal to the standard cell potential E zero, minus 0.0592 over n. Even under non-standard conditions, the cell potentials of electrochemical cells can be determined with the help of the Nernst equation. Nernst Equation is an equation that represents relationship between the concentrations of an ion under non-standard and equilibrium conditions. So when we do that, so we have 0.0257 times the natural log of 10, that gives us 0.0592, so this is equal to 0.0592.

#Nernst equation calculator full#
In electrochemistry, the Nernst equation is an equation that relates the reduction potential of an electrochemical reaction (half-cell or full cell reaction) to the standard electrode potential, temperature, and activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation. This question is of chapter electrochemistry.įormula used: $ $. Hint: We need to calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.


 0 kommentar(er)
0 kommentar(er)
